A new pathway that triggers septic shock has been identified
 Researchers at the University of North Carolina School of Medicine investigated have identified internal sensors that detect bacteria and trigger an alarm that signals for response from the immune system. The fidnings could lead to new therapies for septic shock – a medical condition that occurs when the immune system overreacts to a bacterial infection to such extent that it causes more harm than good.
Researchers at the University of North Carolina School of Medicine investigated have identified internal sensors that detect bacteria and trigger an alarm that signals for response from the immune system. The fidnings could lead to new therapies for septic shock – a medical condition that occurs when the immune system overreacts to a bacterial infection to such extent that it causes more harm than good.
The body’s immune system acts as a home security system, it has sensors on the outside of the cells as well as interior sensors. Exterior sensors act like motion detectors that trigger when there’s a suspicious bacterium seems to be found. For over a decade, researchers have known about one group of external sensors called Toll-like receptors that are used to recognize bacteria.
Identifying a sensor pathway inside cells, the research team showed that both exterior and interior sensors work together to detect the same component of bacterial cell membranes – a molecule called lipopolysaccharide (LPS) also known as endotoxin.
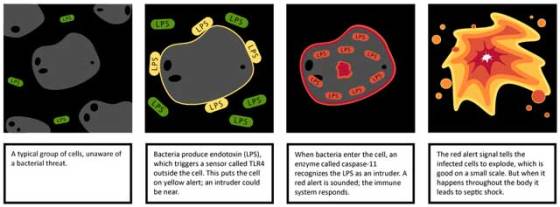
“During the defense against an infection you want to be able to differentiate between the bacteria that stay on the outside of the cell and the ones that get inside”, said Edward A. Miao, MD, PhD, assistant professor of microbiology and immunology. “You can think of the exterior sensors as a yellow alert; they tell us that bacteria are present. But these bacteria could either be simple ones in the wrong place, or very dangerous ones that could cause a serious infection. The interior sensors act as a red alert; they warn us that there are bacteria with ill intent that have the genetic capacity to invade and manipulate our cells.”
Response to a bacterial infection includes an increase in blood vessel permeability near the area under attack, which allows immune system cells to leave the bloodstream and seek and destroy the bacteria. Fluid also leaks into the area surrounding the infection, causing swelling. When the infection spreads through the blood, immune response occur throughout the whole body, blood pressure plummets, overtaxing the heart and leading to organ failure and often death.
This syndrome, known as septic shock, affects millions of people around the world each year. Its most common victims are children, immunocompromised individuals, and the elderly, as their immune systems cannot deal with the infection as effectively as those of healthy adults.
Bacteria that produce LPS cause about half of the cases of septic shock. In fact, much of what is known about endotoxic shock comes from studying animals injected with high doses of LPS. Previous studies pointed the role of the exterior sensor Toll-like receptor 4 gene (TLR4) in regulation of LRS response.
The UNC research team showed that an interior sensor called caspase-11 sounds an alert when bacteria enter a cell. They discovered that this sensor detects the same molecule as the TLR4 sensor.
Through a number of experiments in animal models of sepsis, Miao’s team showed that the exterior and interior sensors work together through a two-step defense mechanism: LPS is first seen on the outside of the cell by TLR4, which sets the interior caspase-11 alarm into a watchful state. At very high doses, the LPS crosses into the cell, tripping the caspase-11 alarm. The result is the generation of the red alert signal, which causes the cell to explode, a form of cell death called pyroptosis. As a consequence, bacteria are deprived of a place to replicate and they are eventually exposed to more potent immune defense.
On the other hand, during sepsis the chain reaction of cells exploding leads to the onset of shock. Future studies will need to figure out how these two sensors get activated in response to a bacterial infection which could help researchers develop new ways of preventing or treating septic shock.
“The septic shock we see in patients is probably a lot more complicated than what we see in this experimental system. The next question we need to ask is whether these same sensors are going off in people with septic shock, and if so, is there a way to block them so we can keep patients from dying”, said Miao.
For more information, read the paper published in the journal Science: “Cytoplasmic LPS Activates Caspase-11: Implications in TLR4-Independent Endotoxic Shock”.









Leave your response!