Injectable shape-memory sponge delivers drugs, cells, and structure
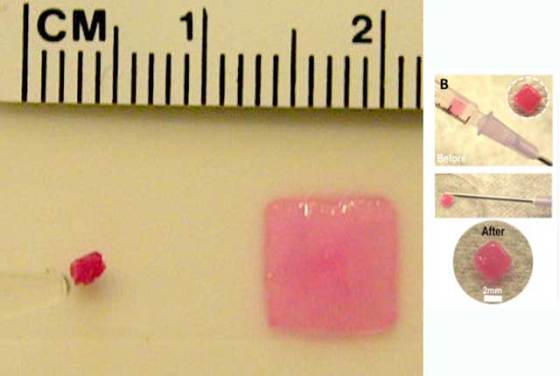
 Harvard University researchers have developed a biocompatible sponge made out of a gel-based material that can be molded into any shape and compressed to a small fraction of its size. This ability allows it to be delivered via injection and restore its original shape and size while gradually releases its cargo. Since it is biocompatible and it has the ability to safely degrade, the technology could be used in a range of minimally invasive therapeutic applications, including regenerative medicine.
Harvard University researchers have developed a biocompatible sponge made out of a gel-based material that can be molded into any shape and compressed to a small fraction of its size. This ability allows it to be delivered via injection and restore its original shape and size while gradually releases its cargo. Since it is biocompatible and it has the ability to safely degrade, the technology could be used in a range of minimally invasive therapeutic applications, including regenerative medicine.
“What we’ve created is a three-dimensional structure that you could use to influence the cells in the tissue surrounding it and perhaps promote tissue formation”, said principal investigator David J. Mooney, Robert P. Pinkas Family Professor of Bioengineering at the Harvard School of Engineering and Applied Sciences (SEAS) and a Core Faculty Member at the Wyss Institute for Biologically Inspired Engineering at Harvard.
Consisting primarily of alginate, a seaweed-based jelly, the injectable sponge contains networks of large pores, which allow liquids and large molecules to easily flow through it. The spongelike gel is formed through cryogelation – a freezing process in which the water in the alginate solution starts to freeze and form pure ice crystals, thus making the surrounding gel more concentrated as it sets. Once the ice crystals melt, a network of pores is left behind.
Unlike other alginate gels which are generally brittle, the researchers tweaked the mixture and the timing of the freezing process to come up with a gel that is extremely strong and compressible.
Mooney and his research team demonstrated that the sponge can hold large and small proteins and drugs within the alginate jelly itself, which are gradually released as the biocompatible matrix starts to break down inside the body. They also demonstrated that this technology could be used to deliver live cells without any damage to the injected cells.
“Our scaffolds can be designed in any size and shape, and injected in situ as a safe, preformed, fully characterized, sterile, and controlled delivery device for cells and drugs”, said Sidi Bencherif, a postdoctoral research associate in Mooney’s lab at SEAS and at the Wyss Institute. “Furthermore, the ability of these materials to reassume specific, pre-defined shapes after injection is likely to be useful in applications such as tissue patches where one desires a patch of a specific size and shape, and when one desires to fill a large defect site with multiple smaller objects. These could pack in such a manner to leave voids that enhance diffusional transport to and from the objects and the host, and promote vascularization around each object.”
The technology could be used to introduce some material into the body to replace tissue that’s been lost or that is deficient, transplant stem cells in order to promote local tissue regeneration, deliver drugs or immune cells, as well as dermal filler in cosmetics. The next goal Harvard researchers have set onto themselves is to perfect the degradation rate of the scaffold in a pace of formation of newly grown tissue in order to increase the number of cells which successfully go though formation process.
For more information, you can read the paper published in the Proceedings of the National Academy of Sciences: “Injectable preformed scaffolds with shape-memory properties” [1.02MB PDF].









Leave your response!