Modified plant protein can be used as drug-delivery vesicle
 Researchers from the University of Pennsylvania (Penn) have developed a new approach for making biocompatible vesicles that can carry drugs to their targets in the body. By starting with a protein that is found in sunflower seeds, they used genetic engineering to make a variety of protein molecules that assemble into biocompatible vesicles and other useful structures with fine tuned shapes.
Researchers from the University of Pennsylvania (Penn) have developed a new approach for making biocompatible vesicles that can carry drugs to their targets in the body. By starting with a protein that is found in sunflower seeds, they used genetic engineering to make a variety of protein molecules that assemble into biocompatible vesicles and other useful structures with fine tuned shapes.
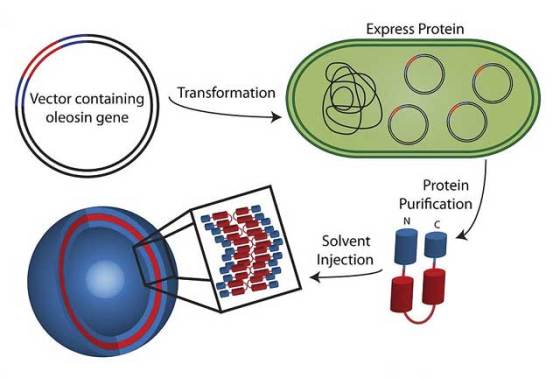
A group led by Daniel A. Hammer, Alfred G. and Meta A. Ennis Professor of Bioengineering, worked for nearly a decade to find a recombinant protein that was biocompatible. Recombinant proteins are the products of a well-established technique that involves introducing a designed gene sequence into a host organism (such as the bacterium E. coli) in order to get that organism to make a protein it would not normally produce.
The molecule the researchers used is called oleosin, and it a surfactant protein found in sunflower and sesame seeds. Surfactants are soap-like chemicals that have two distinct sides – one side is attracted to water and the other is repelled by it – thus enabling it to make many structures in solution.
The team systematically modified oleosin to find variants of the molecule that could form vesicles. Vesicles usually have two walls aligned so the two water-repeling sides face each other. Getting oleosin to take this complex shape meant selectively removing and changing parts of oleosin’s gene sequence so that the corresponding protein would fold the way the researchers wanted after it was produced by the E.coli.
The water-loving interior cavity allows the transport of a large payload of water-soluble molecules that are suspended in water. Since many drugs are water soluble, vesicles offer significant advantages for drug delivery.
“We started by truncating the sequence that codes for the hydrophobic part, shortening the protein itself”, said Hammer. “We did more complex truncations at the ends for separation and to control the shape of the assembly.”
In the process of finding the right protein for this task, the researchers came up with several other useful protein variants that form different shapes, including sheets and fibers, when grown in the appropriate salt solutions.
“There are simple ways to correlate the gene sequence to the geometry you get in the protein”, said graduate student Kevin Vargo, who was a part of the team with research scientist Ranganath Parthasarathy of the Department of Chemical and Biomolecular Engineering in Penn’s School of Engineering and Applied Science. “For example, getting the right amount of curvature to make a spherical vesicle means the chains should be sufficiently large that they do not pack tightly.”
Materials made by recombinant methods offer an additional advantage in that the precise sequence of amino acids can be controlled for targeting to specific receptors and other biological targets. For proteins of this size, this level of control is not attainable by any other method.
Unlike synthesized polypeptide vesicles which are hard to control the sequences in individual sections of their molecules, the vesicles developed by Penn researchers enable a change of a single amino acid in the protein by modifying the corresponding part of the gene. The process also allows consistent creation of polymers that are all of a defined length and dictate the chemical composition at each location along that length.
“The vast majority of our time in this project was doing the imaging; making the protein was relatively easy”, said Hammer.
The imaging technique used is known as cyro-transmission electron microscopy, or cryoTEM. The researchers had to create a thin layer of solution which was plunged into ethane, freezing it fast enough that the water doesn’t crystallize. The cryoTEM technique allows imaging of these structures without the influence of ice crystals that would damage and destroy the vesicles.
Since the chosen protein is already a part of our nutrition, the researchers are confident that their oleosin vesicles will be of great interest in drug-delivery applications, particularly oral-drug delivery. In future development, Penn researchers they plan to add genes for functional groups to allow the vesicles to target certain tissues, as well as determining whether the proteins can be induced to change shape once they reach their targets.
“This research opens up the possibility of using switchable motifs to allow us to release high concentrations of drugs on different cues, such as a change in acidity”, said Hammer. “Tumor microenvironments and the interior of tumors are known to be acidic, so a vesicle that falls apart in acidic environments would be extremely valuable.”
For more information, you can read the paper published in the Proceedings of the National Academy of Sciences: “Self-assembly of tunable protein suprastructures from recombinant oleosin” [1.36MB PDF].









Leave your response!