Mussel biomimicry could lead to new super-strong polymers
 We may like to eat mussels steamed in white wine, but we also like to find mussels at the beach. Researchers at the Max Planck Institute of Colloids and Interfaces and collaborators at the University of California at Santa Barbara and the University of Chicago believe they have uncovered the basis how marine mussels use the byssus, a bundle of tough and extensible fibres, to fasten securely to wave-swept rocky coastlines.
We may like to eat mussels steamed in white wine, but we also like to find mussels at the beach. Researchers at the Max Planck Institute of Colloids and Interfaces and collaborators at the University of California at Santa Barbara and the University of Chicago believe they have uncovered the basis how marine mussels use the byssus, a bundle of tough and extensible fibres, to fasten securely to wave-swept rocky coastlines.
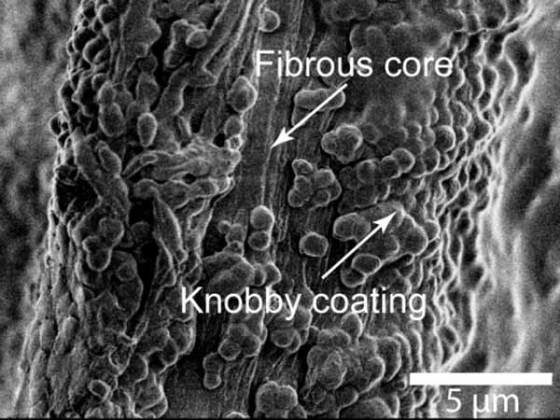
The individual byssal threads that compose the byssus are stiff, but stretchy and are fashioned by the mussel in a process resembling injection molding. Byssal threads are depended upon for dissipating the energy of crashing waves and also for resisting abrasive damage from water-borne debris. To this end, threads are sheathed with a thin and knobby outer cuticle; a biological polymer, which exhibits epoxy-like hardness, while straining up to 100% without cracking.
When viewed under a scanning electron microscope, the byssal cuticles have a knobby appearance. This is because they contain numerous submicron-sized granular inclusions, which are distributed in a continuous matrix. It is believed that when the cuticle is stretched, submicron-sized tears occur in this matrix, hindering the formation of larger cracks.
Central to understanding the peculiar mechanical behaviour of the cuticle are the high concentration of iron ions in the cuticle and the presence of an uncommon modification of the amino acid tyrosine known commonly as dopa. Dopa is found at high concentrations in the main cuticle component, mussel foot protein-1 (mfp-1). Dopa is distinguished from typical amino acids due to its impressive affinity for complexing with transition metal ions, particularly iron. As Admir Masic, a scientist at the Max Planck Institute for Colloids and Interfaces who worked on the project, explains, “when 2-3 dopa residues complex with a single iron ion, they create an incredibly stable complex that can be utilized to cross-link structural proteins.” These metal-protein complexes have a high breaking force (nearly half that of covalent bonds), but unlike covalent bonds they are reversibly breakable, making them ideal for creating sacrificial cross-links.
Using a technique known as in situ Raman spectroscopy to probe the chemical composition of the cuticle, the researchers provided the first direct evidence that the cuticle is a protein-based polymeric scaffold stabilized by dopa-iron complexes. Moreover, it was discovered that the distribution of dopa-iron complexes is clustered, with areas of high density coinciding with the granular inclusions and low density with the inter-granular matrix. These observations, coupled with previous mechanical observations suggest that the densely cross-linked granules function as hard inclusions and the less cross-linked matrix functions in a sacrificial manner, allowing bonds to break prior to catastrophic failure.
“Nature has evolved an elegant solution to a problem that engineers are still struggling with; namely, how to combine the properties of abrasion resistance and high extensibility in the same material”, says Peter Fratzl, director of the biomaterials department at the Max Planck Institute for Colloids and Interfaces. Apparently, the cuticle achieves this through a careful tailoring of protein-metal chemistry and the submicron organization of cross-link density. “Conceivably, this same strategy could be applied in engineered polymers and composites.”









Leave your response!