Nanosensors in hydrogel enable tracking of transplanted cells
 Researches at the Johns Hopkins University School of Medicine have developed a method for in vivo monitoring of transplanted-cell viability. The researchers used nanoscale pH sensors and magnetic resonance imaging (MRI) to track liver cells previously transplanted into mice. This new method is likely to be an important tool for improving cell replacement therapies for conditions such as liver failure and type 1 diabetes.
Researches at the Johns Hopkins University School of Medicine have developed a method for in vivo monitoring of transplanted-cell viability. The researchers used nanoscale pH sensors and magnetic resonance imaging (MRI) to track liver cells previously transplanted into mice. This new method is likely to be an important tool for improving cell replacement therapies for conditions such as liver failure and type 1 diabetes.
“This technology has the potential to turn the human body into less of a black box and tell us if transplanted cells are still alive”, said Mike McMahon, Ph.D., an associate professor of radiology at the Johns Hopkins.
Advances in the field of regenerative medicine depend heavily on the reliability of methods of replacing damaged or missing cells, such as injecting pancreatic cells in people with diabetes. Type 1 diabetes causes a loss of pancreatic beta-cells and insulin dependency since cells don’t make enough insulin. In order to protect the transplanted pancreatic cells from the immune system, they can be placed in hydrogel membrane before transplantation. Nevertheless, these cells eventually stop working in most patients. Then it is assumed that the cells have died, but time and reason of their deaths remain unclear.
The researchers devised an extremely tiny nanosenor composed of amino acid L-arginine and fat, as well as separate indicator molecules that produce signals detectable by Magnetic Resonance Imaging (MRI) as well as light signals when the cells are alive. L-arginine is sensitive to small changes in pH value, which can be caused by the death of nearby cells. Changes in the acidity value would initiate changes in sensor molecules, producing a signal that could be detected by MRI.
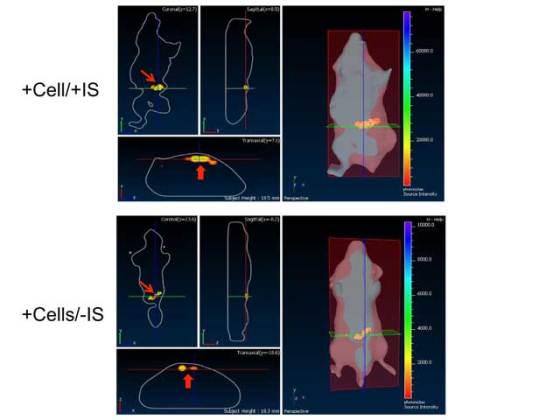
In order to test the efficiency of nanosensors in vivo, the research team placed them into hydrogel spheres along with liver cells and sensory molecules that produce bioluminescent light when the cells are alive. They injected these spheres under the skin of mice. MRI precisely detected the position of these cells in the body, and the researchers were able to observe the number of cells which were still alive. These light indicators cannot be used to check cells in humans because the size of our bodies prevents the visible signals from getting through.
The components of these spheres such as hydrogel membrane, fat molecules and L-arginine are safe for humans. These pH nanosensors can be useful in clinical trials because it could enable doctors to see whether the transplanted cells are alive or dead. It could also lead to better understanding of what’s killing the cells, and how to prevent it.
“Potential applications of the sensors are not limited to cells inside hydrogel capsules. These nanoparticles would work outside capsules, and they could be paired with many different kinds of cells. For example, they may be used to see whether tumor cells are dying in response to chemotherapy”, said Jeff Bulte, Ph.D., the director of cellular imaging at Hopkins’ Institute for Cell Engineering.
For more information, read the article published in Nature: “MRI-detectable pH nanosensors incorporated into hydrogels for in vivo sensing of transplanted-cell viability”.









Leave your response!