A crystalline material with amorphous building blocks
 An international team of scientists led by a researcher from Carnegie Institution of Washington observed the world’s first hybrid crystalline/amorphous material which is capable of indenting diamond. The material is a superhard mixture of crushed carbon spheres and a hydrocarbon solvent, which could find many potential applications for a range of mechanical, electronic, and electrochemical uses.
An international team of scientists led by a researcher from Carnegie Institution of Washington observed the world’s first hybrid crystalline/amorphous material which is capable of indenting diamond. The material is a superhard mixture of crushed carbon spheres and a hydrocarbon solvent, which could find many potential applications for a range of mechanical, electronic, and electrochemical uses.
Carbon is available in a wide variety of forms, including the honeycomb-like graphene, the pencil “lead” graphite, diamond, cylindrically structured nanotubes, and hollow spheres called fullerenes. Some forms of carbon are crystalline (with structure organized in repeating pattern of atomic units), while other forms of carbon are amorphous (with unordered structure). Scientists believed there could be hybrid forms that combine both crystalline and amorphous properties, but they were never experimentally observed.
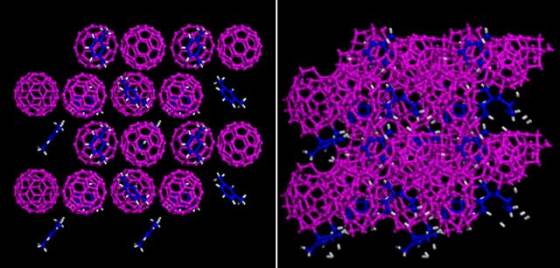
Led by Lin Wang from the Carnegie Institution of Washington, the international team of researchers from the Jilin University, University of Nebraska, Stanford University and Argonne National Laboratory, started with a substance called carbon-60 cages. Carbon-60 cage is made of highly organized balls of carbon constructed of pentagon and hexagon rings bonded together to form a round, hollow shape. The space between the balls of carbon was filled with an organic xylene in order to form a stronger structure.
In order to inspect the properties of the new material, the researchers applied pressure to this combination of carbon cages and solvent. At relatively low pressure, the carbon-60’s cage structure remained, but as the pressure increased, the cage structures started to collapse into more amorphous carbon clusters which formed a lattice structure.
The team discovered that there is a narrow window of pressure (about 320,000 times the normal atmosphere) under which this new structured carbon is created and does not revert back to the cage structure when pressure is removed. In order to create these high-pressure conditions, the researchers used an diamond anvil which was indented during the creation of the newly devised superhard material.
If the solvent used to prepare the new form of carbon is removed by heat treatment, the material loses its lattice periodicity, indicating that the solvent is crucial for maintaining the chemical transition that underlies the new structure. Because there are many similar solvents, it is theoretically possible that an array of similar, but slightly different, carbon lattices could be created using this pressure method.
“We created a new type of carbon material, one that is comparable to diamond in its inability to be compressed”, said Wang, High Pressure Synergetic Consortium (HPSynC), Geophysical Laboratory, Carnegie Institution of Washington. “Once created under extreme pressures, this material can exist at normal conditions, meaning it could be used for a wide array of practical applications.”
For more information, read the paper published in the journal Science: “Long-Range Ordered Carbon Clusters: A Crystalline Material with Amorphous Building Blocks”.
For more information about the materials and methods of the research, you can read the supplementary information provided by the researchers [2.47MB PDF].









Leave your response!