A foldable ceramic that is also electrically conductive
 Cooperation between researchers at Stuttgart University, the Max Planck Institute for Intelligent Systems and the Max Planck Institute for Solid State Research, resulted with a material which is as hard as copper, yet flexible enough to be rolled up or folded. This material is actually made from a vanadium pentoxide ceramic, and it has another property which makes it unlikable to other ceramics – it is electrically conductive.
Cooperation between researchers at Stuttgart University, the Max Planck Institute for Intelligent Systems and the Max Planck Institute for Solid State Research, resulted with a material which is as hard as copper, yet flexible enough to be rolled up or folded. This material is actually made from a vanadium pentoxide ceramic, and it has another property which makes it unlikable to other ceramics – it is electrically conductive.
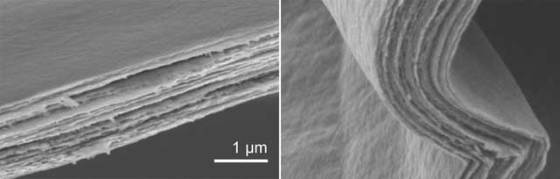
The ceramic paper’s special mechanical properties are derived from its structure, which resembles that of mother-of-pearl. Mollusk shells are hard but brittle aragonite platelets are stacked in layers like bricks and joined using a protein “mortar”, thus creating the hard, yet elastic and sturdy mother-of-pearl.
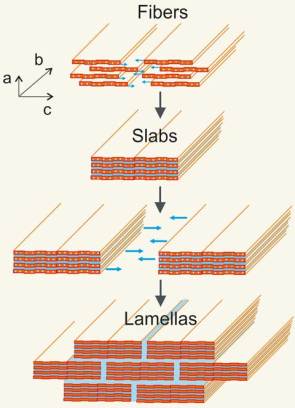
Researchers produced the ceramic paper consisting of conductive nanofibers of vanadium pentoxide in a straightforward and simple way. First, they synthesized vanadium pentoxide nanofibers by using water-soluble vanadium salt. The rather unusual feature of this ceramic is that the fibers conduct electricity. This is possible because the metal oxide chains contain weakly bound electrons which can hop along them.
In order to assemble these nanofibers into strong and elastic material, the researchers distributed the nanofibers suspended in water very thinly on a substrate, and afterwards let the aqueous film dry for several hours at room temperature. The next step is to expose them for a few more hours at temperature of 40°C (104°F) in order to slowly reduce the humidity in the climate chamber. This slow process allowed the fibers to assemble themselves into precisely parallel patterns. In final step, the resulting film is heated to 100 and 150°C (212 and 302°F), thus producing a transparent, orange paper-like material.
The thickness of the material can be altered by changing the amount of the used nanofiber solution, and the researchers created the material with thickness between 0.5 and 2.5 micrometers. The resulting ceramic paper is not only more elastic than mother-of-pearl, it is also harder. What is more, it conducts electricity.
“The paper can be folded like an accordion or rolled up”, said Dr. Žaklina Burghard, Institute of Material Science at Stuttgart University. “However, the conductivity along the paper fibers is much greater than across them.”
The reason for the varying conductivity of the paper depending on the direction in which the scientists measure it, also explains its remarkable mechanical properties. They are both a result of the material’s structure. The fibers consist of two vanadium pentoxide layers with a layer of water in between. Several fibers stack on top of one another laterally, forming slabs. The slabs also stack laterally, but staggered, on top of each other, so that the structure of the layered material will likely resemble a brick wall in a cross-section, where the vanadium pentoxide slabs make up the bricks embedded in a water layer that surrounds them like mortar.
It is this combination of hard ceramic and soft water in the special nanostructure that makes the paper hard, strong and pliable. It also results in high conductivity in the paper plane and low out-of-plane conductivity. However, the electricity is not only transported by the electrons that move along the nanofibers, but also by ions in the water layers between the ceramic.
Both the electrical properties and the mechanical properties of the paper therefore vary according to the water content. By drying and annealing the material, the scientists mainly remove weakly bound water to make the ceramic fibers form a denser structure. Since this also reinforces the bonds between the nanofibers, it makes the paper harder and more rigid.
Since the paper is structured in regular and homogeneously shaped layers, ions can move efficiently in a particular in-plane direction, making the material suitable as electrode material for batteries. Batteries with ceramic paper electrodes could therefore be charged quickly, but also discharged quickly to allow for high current densities. Industry is already showing a keen interest in using the paper in rechargeable batteries.
Since electrons can be made more mobile in vanadium oxide thanks to molecular interaction, it is also suitable for gas sensors. In addition, the ceramic paper could give life to artificial muscles. When foreign ions accumulate in the composite, it expands. As an actuator controlled by the number of intercalated particles, the ceramic paper could push or pull objects down to microscopic size. Max Planck researchers want to increase the usefulness of the ceramic paper by combining it with other materials to create even more versatile and better properties.
For more information, read the paper published in Advanced Materials: “Hydrogen-Bond Reinforced Vanadia Nanofiber Paper of High Stiffness“.









Leave your response!