A nanoscale insight on lithium batteries could increase battery durability
 Battery developers know that recharging and using lithium batteries over and over damages the electrode materials. Researchers at the DOE’s Environmental Molecular Sciences Lab on the Pacific Northwest National Lab (PNNL) grounds have been observing how nanowires composed of tin oxide rapidly change shape and deform when they are being charged.
Battery developers know that recharging and using lithium batteries over and over damages the electrode materials. Researchers at the DOE’s Environmental Molecular Sciences Lab on the Pacific Northwest National Lab (PNNL) grounds have been observing how nanowires composed of tin oxide rapidly change shape and deform when they are being charged.
“Nanowires of tin oxide were able to withstand the deformations associated with electrical flow better than bulk tin oxide, which is a brittle ceramic”, said Chongmin Wang, a materials scientist at the lab. “It reminds me of making a rope from steel. You wind together thinner wires rather than making one thick rope.”
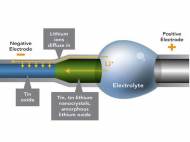
Wang, PNNL chemist Wu Xu and Jianyu Huang from the Center for Integrated Nanotechnologies, Sandia National Lab, built a small battery with lithium cobalt oxide as the anode and a cathode composed of a single tin oxide nanowire. The team used a specially outfitted transmission electron microscope (TEM) to set up a miniature battery. This instrument allowed them to image smaller wires of about 200 nanometers in diameter (about a fifth the width of the previous nanowires) while they charged it.
The miniature battery included a positive electrode of lithium cobalt oxide and a negative electrode made from thin nanowires of tin oxide. Between the two electrodes, an electrolyte provided a conduit for lithium ions and a barrier for electrons. The electrolyte was specially designed to withstand the conditions in the TEM. When the team charged the miniature battery at a constant voltage, the wire twisted and increased its total volume by about 250 percent.
Apparently, the ions cause a reaction front to move through the nanowire creating a moving “medusa zone” or cloud of dense dislocations. These dislocations are nucleated and absorbed at the moving front.
The researchers noted that the nanowire is in a crystalline state at the beginning, but the ions convert the tin oxide into a glassy state. They think the amount of accumulated deformation might provide a clue as to why some of these battery materials wear down. However, they also note that tin oxide nanowires perform better than bulk tin oxide cathodes.
The reason that atomic-scale examination of the charging and discharging process of a single nanowire had not been possible was because the high vacuum in a TEM made it difficult to use a liquid electrolyte. Part of the Huang group’s achievement was to demonstrate that a low-vapor-pressure ionic liquid (essentially, molten salt) could function in the vacuum environment.
“The methodology that we developed should stimulate extensive real-time studies of the microscopic processes in batteries and lead to a more complete understanding of the mechanisms governing battery performance and reliability”, said Huang. “Our experiments also lay a foundation for in-situ studies of electrochemical reactions, and will have broad impact in energy storage, corrosion, electrodeposition and general chemical synthesis research field.”










Good innovation for battery durability.
Dr.A.Jagadeesh Nellore(AP),India