Advancement in complex 3D polymer brush nanostructures creation
 Polymer brushes are polymers in which individual polymer chains stand side by side on a surface, causing the chains to stick out like bristles on a brush. A team of researchers at the University of California, Santa Barbara, and The Dow Chemical Company (Midland, Michigan) have developed a new method that allows creation these three-dimensional nanostructures in a controlled fashion from polymer brushes.
Polymer brushes are polymers in which individual polymer chains stand side by side on a surface, causing the chains to stick out like bristles on a brush. A team of researchers at the University of California, Santa Barbara, and The Dow Chemical Company (Midland, Michigan) have developed a new method that allows creation these three-dimensional nanostructures in a controlled fashion from polymer brushes.
Although there are efforts to create new brush structures, current methods do not offer sufficient temporal and spatial control over the growth process. Current methods usually employ a self-organized monolayer of an initiator which is assembled on a substrate and the polymer chains can grow out from there. In order to obtain desired patterns, the initiator must be applied to the substrate in a corresponding pattern. However, this process is difficult and does not allow generation of complex 3D structures.
Led by Craig J. Hawker, researchers at the UCSB achieved formation of brushes on a uniform initiator layer with both spatial and temporal control. Their simple method is based on a light-activated radical polymerization. The length of the bristles at any given location depends only on the duration and intensity of the local irradiation.
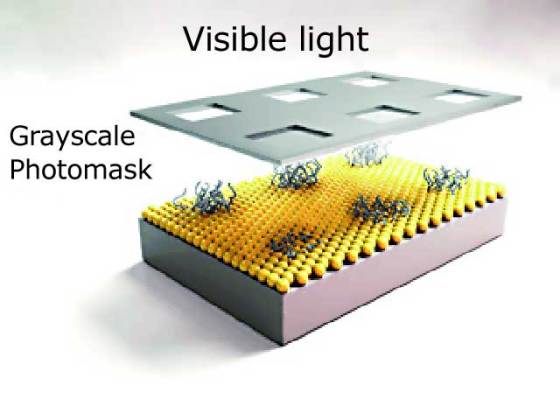
To form a specific structure, conventional photomasks can be used. Photomasks have openings in the areas to be irradiated and shield the other areas from the light. Combined with a special iridium-based photocatalyst, this method allows formation of extensive patterns with submicrometer resolution in one step. The photocatalyst remains active for only a very short time after irradiation, so it cannot travel very far into nonirradiated areas while in its active state. It is even possible to use a grayscale photomask with continuously increasing opacity to produce gradated patterns.
Another advantage of the new method developed by Hawker Research Group UCSB is that newly incorporated monomers are always added to the chain adjacent to the initiator, meaning that the initiator remains at the forward end of the growing chain.
Because it is not destroyed as in other methods, and remains available at the right position, the polymerization can be stopped and restarted at any time. In this way the mask being used can be exchanged as often as desired. It is even possible to vary the monomer being used during the process. The complexity of accessible structures and applications is thus almost unlimited.
There are a wide variety of current and future applications for polymer brushes. For example, a coating of polymer brushes on a plastic surface such as an artificial heart valve or a dialysis machine can hinder the adsorption of proteins onto the surface. It can also be used in the fabrication of next-generation microelectronic devices. Other areas of application include biocompatible coatings for implants, chemical sensors, and new “intelligent” materials.
For more information, read the paper published in Angewandte Chemie: “Fabrication of Complex Three-Dimensional Polymer Brush Nanostructures through Light-Mediated Living Radical Polymerization”.









Leave your response!