Graphene ‘onion rings’ – creating graphene nanoribbons atom by atom
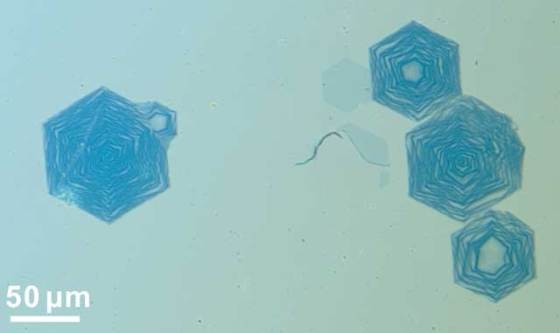
 Experiments at Rice University meant to reveal how graphene grows under high pressure and in a hydrogen-rich environment resulted up with development of concentric hexagons of graphene. The process represents the first time anyone has synthesized graphene nanoribbons atom by atom on metal. Once perfected, the approach could lead to breakthroughs in electronics lithium ion batteries.
Experiments at Rice University meant to reveal how graphene grows under high pressure and in a hydrogen-rich environment resulted up with development of concentric hexagons of graphene. The process represents the first time anyone has synthesized graphene nanoribbons atom by atom on metal. Once perfected, the approach could lead to breakthroughs in electronics lithium ion batteries.
Usually, graphene grown in a hot furnace by chemical vapor deposition starts on a speck of dust or a bump on a copper or other metallic surface. This speck is referred to as seed since it causes a carbon atom to latch onto it and start forming the chicken-wire grid structure in a process called nucleation. The challenge was to figure out how such a thing could grow.
Rice chemist James Tour, Rice theoretical physicist Boris Yakobson and their teams found that the entire edge of a fast-growing sheet of graphene becomes a nucleation site when treated with hydrogen (hydrogenated). The edge lets carbon atoms get under the graphene skin, where they start a new sheet. But because the top graphene grows so fast, it eventually halts the flow of carbon atoms to the new sheet underneath. The bottom stops growing, leaving a graphene ring. Then the process repeats.
“The mechanism relies on that top layer to stop carbon from reaching the bottom so easily”, said Tour. “What we get are a multiple of single crystals growing one on top of the other.”
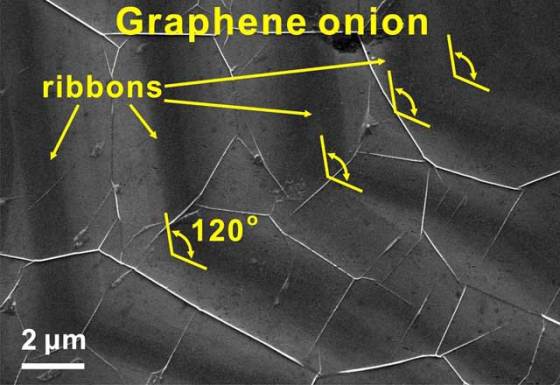
As we reported back in 2009, Tour lab pioneered the bulk manufacture of single-atom-thick graphene nanoribbons with the discovery that carbon nanotubes could be chemically “unzipped” into long sheets. Instead cutting down larger sheets into ribbons like in their previous research, growth of a ribbon from the bottom up enables control of the edges.
Graduate student Zheng Yan, a member of Tour’s group and lead author of the research paper concerting this methodology, discovered the new way to create nanoribbons while experimenting with graphene growth under hydrogen pressurized to varying degrees.
The atomic configuration at the edge helps determine graphene’s electrical properties. By changing relative pressures of the growth environment of hydrogen versus carbon, you can create entirely new structures which are significantly different from regular graphene.
The sweet spot for creation of rings was at 66,661 Pa (500 Torr). The edges of hexagonal graphene onion rings are zigzags, which make the rings metallic. The width of the rings, which ranged from 10 to 450 nanometers, also affects their electronic properties, and the researchers plan to find a way to control the production process to be more consistent.
Yakobson’s group confirmed that the microscopic rings formed underneath and not on top of the sheet through first-principle calculations. Yan also determined the top sheet of graphene could be stripped away with argon plasma, leaving stand-alone rings.
Nanoribbons are being studied for use in batteries and advanced electronics and as heat sinks. Once the Rice researchers perfect the production process, they could turn them into low-voltage transistors. They could also be suitable for lithium storage in next-generation of lithium ion batteries.
For more details, read the paper published in Journal of American Chemical Society: “Hexagonal Graphene Onion Rings”.









Leave your response!