More energy efficient microscale method for the desalination of seawater
 Collaboration between engineers at the University of Texas at Austin and the University of Marburg in Germany resulted in the development of a new method for the desalination of seawater. In comparison with conventional techniques, the new method is significantly simpler and requires less energy. Energy consumption is so little that it can work with a simple battery.
Collaboration between engineers at the University of Texas at Austin and the University of Marburg in Germany resulted in the development of a new method for the desalination of seawater. In comparison with conventional techniques, the new method is significantly simpler and requires less energy. Energy consumption is so little that it can work with a simple battery.
The availability of fresh water for drinking as well as crop irrigation is crucial for maintaining and improving human health. Even though water covers 70 percent of the Earth’s surface, most of it is saline. Unlike current methods for desalinating water which rely on costly and easily contaminated membranes, the research team has developed membrane-free technique called electrochemically mediated seawater desalination which separates salt from water at a microscale.
This new method for seawater desalination holds promise for the water-stressed areas in which about a third of the planet’s inhabitants live. Despite having access to abundant seawater, these areas often don’t have required infrastructure or finances necessary to desalt water using expensive conventional technology.
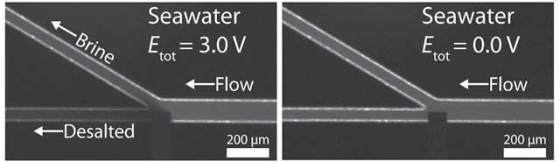
The new method presses the sea water by a system of two microchannels, each 22 microns wide. These two small channels – auxiliary channel and the working channel – are connected electrically via a bipolar electrode. The auxiliary channel is connected to a voltage source and the working channel is grounded, thus creating a potential difference of 3.0V between the channels.
The working channel has two branches and an embedded electrode at the branch point. This electrode neutralizes some of the chloride ions in seawater to create a zone depleted of negatively charged ions that prevent the salt from passing through. As a result, a local electric field is increased compared with the rest of the channel. This change in the electric field is sufficient to redirect salts into one branch, allowing partially desalinated water to pass through the other branch.
According to researchers, the neutralization reaction at the electrode is crucial for the desalination. Currently, this method achieves 25 percent desalination, but the researchers are confident that it could meet the goal of 99 percent desalination which is needed to create potable water. Also, this technique needs to be scaled up because currently used microchannels produce about 40 nanoliters of desalted water per minute. In order to make this method practical for commercial use, it would have to produce liters of water per day.
“The membrane-free method we’ve developed still needs to be refined and scaled up, but if we can succeed at that, then one day it might be possible to provide fresh water on a massive scale using a simple, even portable system”, said Richard Crooks the Robert A. Welch Chair in Materials Chemistry at the University of Texas at Austin.
It’s patent-pending and is in commercial development by startup company Okeanos Technologies.
For more information, read the article published in the journal Angewandte Chemie: “Electrochemically Mediated Seawater Desalination“.









Leave your response!