New catalyst turns C-H bonds to C-Si bonds at a high rate
 A team of researchers from the University of Delaware (UD) have reported their findings about a new chemical reaction that uses palladium as a catalyst in a process where carbon-hydrogen bonds are converted to carbon-silicon bonds. The process could lead to development of new materials, and it has a huge potential in development and manufacture of new medicines and plastics.
A team of researchers from the University of Delaware (UD) have reported their findings about a new chemical reaction that uses palladium as a catalyst in a process where carbon-hydrogen bonds are converted to carbon-silicon bonds. The process could lead to development of new materials, and it has a huge potential in development and manufacture of new medicines and plastics.
Led by assistant professor Donald Watson, the UD Department of Chemistry and Biochemistry team was inspired to use the metal palladium by previous work of Richard F. Heck, UD Willis F. Harrington Professor Emeritus, who won the 2010 Nobel Prize in Chemistry alongside co-laureates Ei-ichi Negishi and Akira Suzuki for discoveries using the metal palladium as a catalyst to get carbon atoms to link together.
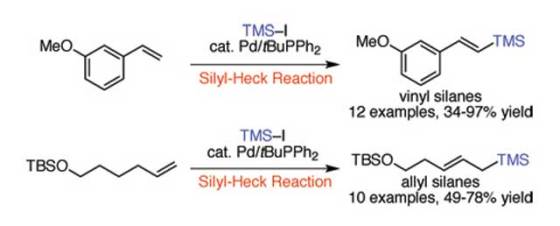
The team came up with a mild palladium-catalyzed reaction which currently takes from 6 to 24 hours to convert the carbon-hydrogen (C-H) bond of an alkene to a carbon-silicon (C-Si) bond. The team examined the early 1990s reports of Japanese scientists, where they managed to achieve a similar chemical reaction with lower yield of 10 to 45 percent, and managed to develop a catalyst that provides above a 95 percent yield.
In order to achieve this improvement, the UD team of researchers created a more reactive “hybrid” catalyst which combines the features of catalysts developed by Heck with more modern systems. Although their first attempts didn’t work, they eventually found that the tert-butyldiphenylphosphine ligand has just the right properties to increase the yield. They modified the reaction conditions from the original research to use the more affordable chlorotrimethylsilane instead iodotrimethylsilane as the silane source.
“We’re excited – we think there are a lot of things we can do with this, and we’re very actively continuing our explorations”, said Watson. “My students did a fantastic job – they really knocked this out of the park.”
He credits doctoral students Jesse McAtee and Sara Martin for their primary contributions to this latest effort, as well as undergraduate students Derek Ahneman and Keywan Johnson who assisted with the research. The Donald A. Watson Research Group is now examining the details of the side reactions and searching for new chemical reactions that could be useful in development of new materials.
“It provides access to new compounds in a very useful, straightforward, economical fashion”, said Watson. “Silicon is one row below carbon on the Periodic Table, so we’re activating a way to move down the table.”
For more information, read the article published in the Angewandte Chemie International Edition: “Preparation of Allyl and Vinyl Silanes by the Palladium-Catalyzed Silylation of Terminal Olefins: A Silyl-Heck Reaction”.









Once they perfect the process we could expect various tailored plastics designed with diverse combinations of characteristics.