Outperforming platinum with a metal-free fuel cell catalyst
 Collaboration between researchers in South Korea, Case Western Reserve University and University of North Texas resulted with a discovery of an inexpensive and easily produced catalyst that performs better than platinum in oxygen-reduction reactions. The finding is a step toward eliminating what industry regards as the largest obstacle to large-scale commercialization of fuel cell technology.
Collaboration between researchers in South Korea, Case Western Reserve University and University of North Texas resulted with a discovery of an inexpensive and easily produced catalyst that performs better than platinum in oxygen-reduction reactions. The finding is a step toward eliminating what industry regards as the largest obstacle to large-scale commercialization of fuel cell technology.
Like a battery, a fuel cell converts chemical energy into electrical energy. It works by removing an electron from a fuel, usually hydrogen or methanol mixed with water, at the cell’s anode, or positive electrode, creating a current. Hydrogen ions produced then pass through a membrane to the cathode, or negative electrode. Here, oxygen molecules from the air are split and reduced by the addition of electrons and combined with the hydrogen ions to form water and heat.
Fuel cells can be more efficient than internal combustion engines, silent, and at least one type produces zero greenhouse emissions at the tail pipe. Car and bus manufacturers as well as makers of residential and small-business-sized generators have been testing and developing different forms of fuel cells for more than a decade but the high cost, limited electrocatalytic activity, poor cycle stability, and sometimes environmental hazard represent hurdles for a broader use of the system.
“We made metal-free catalysts using an affordable and scalable process”, said Liming Dai, the Kent Hale Smith Professor of macromolecular science and engineering at Case Western Reserve. “The catalysts are more stable than platinum catalysts and tolerate carbon monoxide poisoning and methanol crossover.”
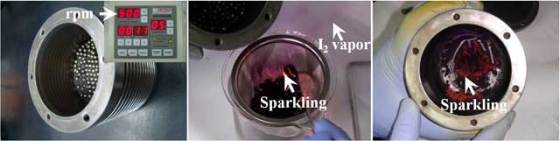
Led by Jong-Beom Baek, director of the Interdisciplinary School of Green Energy/Low-Dimensional Carbon Materials Center at South Korea’s Ulsan National Institute of Science and Technology, researchers coated cathode with graphene nanoparticles edged with iodine instead platinum catalyst. Their mix proved more efficient in the oxygen reduction reaction, generating 33 percent more current than a commercial cathode coated with platinum.
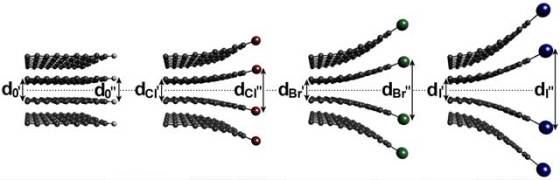
The technology to make alternative catalysts relies on a simple and cheap industrial process several of the researchers developed to make graphene sheets from graphite. Graphite is placed inside a ball miller, which is a canister filled with stainless steel balls with high speed rotation (500 rpm), where it is being milled into single-layer graphene nanoparticles. Chlorine, bromine or iodine gas is injected while the canister is turning in order to form different catalysts. Gas molecules replace carbon atoms along the zigzag edges of nanoplatelets created by milling.
Aside creating edges with a pattern favorable to binding with oxygen molecules, the method also lowered the bond strength between the two oxygen atoms. The weaker the oxygen bonds became, the more efficiently the oxygen was reduced and converted to water at the cathode.
In their experiments, researchers found that a cathode coated with iodine-edged nanoplatelets performed best. A cathode coated with bromine-edged nanoparticles generated 7 percent less current than the commercial cathode coated with platinum, the chlorine-edged nanoplatelets 40 percent less. Electrodes coated with the nanoplatelets managed to outperform platinum electrodes by maintaining 85.6 percent to 87.4 percent of their initial current after 10,000 cycles. In comparison, commercial platinum electrodes maintain only 62.5 percent.
Carbon monoxide was added to replicate the poisoning that many scientists blame for the poor performance of platinum as a catalyst. The performance of the graphene-based catalysts was unaffected. When methanol was added to replicate methanol crossover from the anode to cathode in direct methanol fuel cells, the current density of the platinum catalyst dropped sharply. Again, the graphene-based catalysts were unaffected.
The results suggest new insights and practical methods for designing edge-functionalized graphene nanoplatelets as high-performance metal-free catalysts. Along with a simple production method which is low-cost and can be scaleable, previously mentioned advantages are promising for applications in fuel cells and other energy-related devices. Case Western Reserve University, University of North Texas and South Korea’s Ulsan National Institute of Science and Technology researchers are now working on optimization of the materials.
For more information, you can read the paper published in Nature’s Scientific Reports: “Facile, scalable synthesis of edge-halogenated graphene nanoplatelets as efficient metal-free eletrocatalysts for oxygen reductionreaction” [1.2MB PDF].









Leave your response!