The potential of rechargeable batteries based on fluoride shuttle
 Lithium-ion batteries have found use in various portable devices, but their storage capacity limits the usability of those gadgets. As we mentioned in a couple of our previous articles, we need a new battery systems capable to charge faster and store more energy. Researchers from the Karlsruhe Institute of Technology (KIT) found a potential solution which relies on fluoride.
Lithium-ion batteries have found use in various portable devices, but their storage capacity limits the usability of those gadgets. As we mentioned in a couple of our previous articles, we need a new battery systems capable to charge faster and store more energy. Researchers from the Karlsruhe Institute of Technology (KIT) found a potential solution which relies on fluoride.
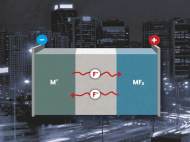
Developed by Dr. Maximilian Fichtner, Head of the Energy Storage Systems Group, and Dr. Munnangi Anji Reddy at the KIT Institute of Nanotechnology (INT), the new concept for rechargeable batteries eliminates the need for usage of lithium. It operates through fluoride shuttle in which the fluoride is used to transfer its anions between the electrodes.
The new lithium-free batteries are made of a fluoride-containing electrolyte, a metal anode, and metal fluoride cathode. In the charging process, metal fluoride is formed at the cathode. During the discharge process, fluoride anions transfer the energy onto the anode.
“As several electrons per metal atom can be transferred, this concept allows extraordinarily high energy densities – up to ten times as high as those of conventional lithium-ion batteries”, said Fichtner.
The weight of these batteries is also lower compared to lithium-ion batteries, and the fact there is no lithium used during the production increases their operational safety. Before you start celebrating the new era of more powerful and practical portable devices, you’ll be disappointed by a few “kinks” that need to be sorted out before this technology meets the market.
The major “flaw” of this technology lies in the very electrolyte itself. The solid electrolyte used in the prototype is suited for applications at elevated temperatures which actually enable it to transfer higher amount of anions. The KIT researchers are trying to find a liquid electrolyte that is suitable for use at room temperature, thus making it usable in portable devices.
There are more problems which are related to the initial capacity and cyclic stability of the fluoride-ion battery, but those problems are solvable by further development of the material design and battery architecture.
For more information, read the article published in the Journal of Materials Chemistry named: “Batteries based on fluoride shuttle”.









Leave your response!