Unexpected antifreeze properties found in a simple compound
 A chemical compound used to stabilize particles in suspension has proved capable of controlling the growth of ice crystals. Unlike the macromolecules previously known for their antifreeze properties, the compound in question is a simple molecule which offers many advantages, including low production costs, stability and ease of use. The research could also lead to development of synthetic equivalents of antifreeze proteins.
A chemical compound used to stabilize particles in suspension has proved capable of controlling the growth of ice crystals. Unlike the macromolecules previously known for their antifreeze properties, the compound in question is a simple molecule which offers many advantages, including low production costs, stability and ease of use. The research could also lead to development of synthetic equivalents of antifreeze proteins.
The formation of ice crystals can have multiple, and often destructive, consequences. Cell degradation in living organisms, or damage to land and roads in cold climates. Many organisms and species that live in cold environments have adapted to control ice growth. Their resistance to low temperatures is based on the presence of antifreeze proteins, all of which are made up of very long organic chains with amphiphilic structures (partly hydrophilic, partly hydrophobic).
Researchers are trying to identify the mechanism enabling antifreeze proteins to identify these crystals, but the phenomenon is still not fully understood. In addition, since these proteins are extremely costly to extract, the preferred solution is to create synthetic equivalents inspired by natural structures. All proteins currently known for their antifreeze properties are macromolecules (like glycoproteins or polysaccharides).
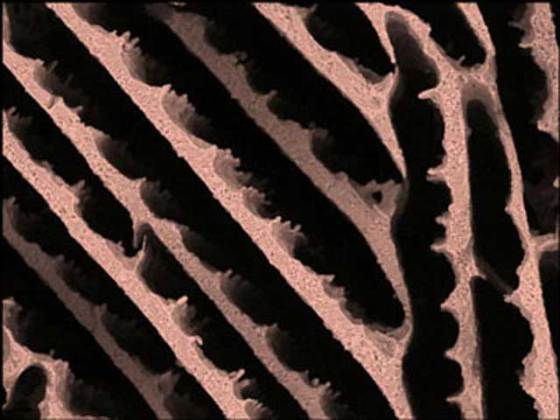
The team led by Sylvain Deville, CNRS researcher at the Laboratoire de Synthèse et Fonctionnalisation des Céramiques (LSFC), used X-ray diffraction and imaging on the X-ray synchrotron (beam line ID19) at the ESRF in Grenoble, France, to discover that zirconium acetate (a chemical compound normally used to stabilize particles in suspension) can be used to control the ice crystal growth.
The compound governs the morphology of the ice crystals obtained by freezing a solution in which it is combined with water. The crystals obtained when adding zirconium acetate are very homogenous, whereas those obtained without it show no particular uniformity.
These results are quite surprising, given that zirconium acetate is an ionic compound made up of cations and anions and forms a neutral product with no net charge. It wasn’t known as a substance capable of controlling ice crystal growth by controlling their morphology. Since this implies a direct interaction with the ice crystals, the researchers were surprised to find out that such radically different molecules as proteins used to control ice crystal growth and zirconium acetate show similar properties when it comes to crystalline growth.
According to researchers, this compound offers significant advantages over both synthetic and natural existing equivalents. It is cheap to produce, stable, “simple” and easy to use, which bodes well for a wealth of future industrial applications. Since it has been shown that an unexpected substance has antifreeze properties, the research could lead to new breakthroughs with even more efficient substances.
For more information, you can read the article published in PLoS ONE named: “Ice Shaping Properties, Similar to That of Antifreeze Proteins, of a Zirconium Acetate Complex”.









I’m impressed, I must say. Rarely do I encounter a blog that’s both educative and amusing, and without a doubt, you’ve hit the nail on the head. The issue is something which too few folks are speaking intelligently about. Now i’m very happy I stumbled across this in my search for something relating to this.