Using high pressure to create better hydrogen storage
 An international team of researchers has synthesized a new material able to store up to three times more hydrogen compared to most known metal hydrides. The iridium hydride from hydrogen and metallic iridium has an unexpected structure that does not occur in other known hydrides. The new structure could inspire creation of new metal hydrides suitable for industrial hydrogen storage.
An international team of researchers has synthesized a new material able to store up to three times more hydrogen compared to most known metal hydrides. The iridium hydride from hydrogen and metallic iridium has an unexpected structure that does not occur in other known hydrides. The new structure could inspire creation of new metal hydrides suitable for industrial hydrogen storage.
Edinburgh University (UK), Oviedo University (Spain) and DESY (Germany) researchers tested various materials under pressure and used intense and bundled X-rays to reveal structural changes with varying pressure. After placing a piece of iridium inside a pressure cell, known as diamond anvil cell, it was loaded with hydrogen. The research team then compressed the sample using pressures up to 125 gigapascals (GPa). In comparison, a single GPa corresponds to 10,000 Earth’s atmospheric pressures.
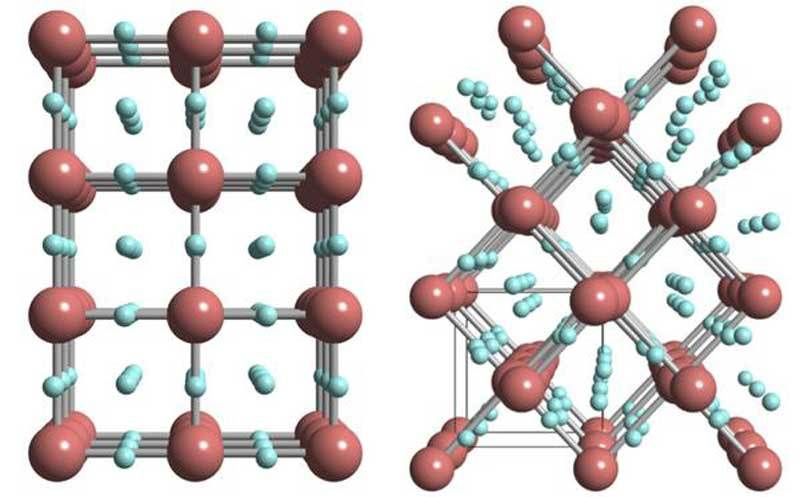
At 55 GPa, the researchers at the found presence of X-ray signals which do not originate from metallic iridium and which became stronger and stronger with increased pressure. Normally, the formation of metal hydrides involves the widening of a metal’s inner structure with hydrogen occupying the intermediate space between metal atoms. In these hydrides, hydrogen is not bound to the metal and typical hydrogen-to-metal ratios are close to one.
As a result, each iridium atom is surrounded by three hydrogen atoms, resulting in an iridium trihydride phase that can store up to three times more hydrogen than most other metal hydrides. Since hydrogen is nearly invisible to X-ray scanning, the researchers had to perform additional theoretical calculations, which supported the presence of a simple cubic lattice, albeit distorted, in iridium hydride.
The researchers concluded that iridium hydride had indeed formed as a new material phase. However, the signals of the hydride were obscured by signals from residual iridium still present in the sample. They solved this problem with laser heating of the sample. Laser heating enabled them to synthesize iridium hydride much faster without leaving residual iridium behind.
Although results from DESY PETRA III’s experimental station P02 for extreme conditions research look promising, bear in mind that iridium is too rare and too expensive for industrial applications. However, the researchers hope a similar approach might reveal more abundant materials that could serve as high-capacity metal hydrides.
“In a broader sense, our research aims at a deeper understanding of how metal hydrides form and how the hydrogen can subsequently be extracted again”, said Thomas Scheler, the study’s first author and former PhD student of Eugene Gregoryanz’s group at Edinburgh University.
For more information, read the paper published in Physical Review Letters: “High-Pressure Synthesis and Characterization of Iridium Trihydride”.









Hydrogen storage is critical and as such this innovative proposal looks bright.
Dr.A.Jagadeesh Nellore(AP),India